Friedel–Crafts alkylation reaction with fluorinated alcohols as hydrogen-bond donors and solvents†
Abstract
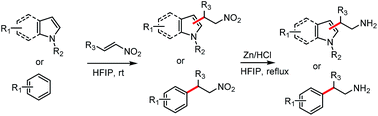
An effective and clean FC alkylation of indoles and electron-rich arenes with β-nitroalkenes in HFIP was reported. The desired products are formed rapidly in excellent yields under mild conditions without the need for any additional catalysts or reagents. Further, this methodology can be applied to one-pot synthesis of biologically active tryptamine derivatives.


 Please wait while we load your content...
Please wait while we load your content...