An efficient and scalable synthesis of potent TLR2 agonistic PAM2CSK4†
Abstract
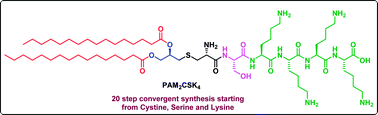
Diacylated PAM2CSK4, a highly expensive lipopeptide with desirable aqueous solubility and a broad spectrum of cytokine/chemokine induction is a most potent dual (human and murine) Toll-Like Receptor-2 (TLR2) agonist. Besides such thrilling characteristics, its synthetic process is not reported in the literature. The present report describes an efficient and scalable 20 step synthesis of PAM2CSK4 in good yield (all steps > 60%) along with a clear description of the hindrances and easy solutions adopted in each step. Overall, a convergent synthetic approach was adopted involving synthesis of appropriately protected starting materials, synthesis of a key backbone skeleton PAM2CS, synthesis of a tetralysine fragment and the final coupling to yield PAM2CSK4. Tedious column chromatography was avoided on a large scale in many steps.


 Please wait while we load your content...
Please wait while we load your content...