The effect of sintering process on lithium ionic conductivity of Li6.4Al0.2La3Zr2O12 garnet produced by solid-state synthesis
Abstract
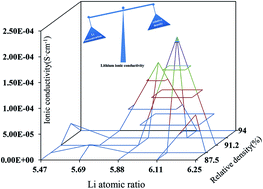
Recently, solid-state electrolyte lithium lanthanum zirconium oxide garnet (Li7La3Zr2O12, LLZO) has attracted great attention due to its high room temperature conductivity of lithium ions and stability against lithium metal electrodes. The Al-doped cubic garnet Li6.4Al0.2La3Zr2O12 was synthesized by a conventional solid-state method at different sintering temperatures. The influence of the sintering process on the structure and ionic conductivity was investigated by X-ray diffraction, electrochemical impedance spectroscopy, and scanning electron microscopy. The results showed that Li vaporization and relative density were affected by the sintering process. The synergistic effects of Li concentration and relative density determined the Li+ ionic conductivity. Compared with the relative density, the Li concentration plays a more important role in determining the ionic conductivity via the solid-state method.


 Please wait while we load your content...
Please wait while we load your content...