A sustainable strategy for the synthesis of bis-2-iminothiazolidin-4-ones utilizing novel series of asymmetrically substituted bis-thioureas as viable precursors†
Abstract
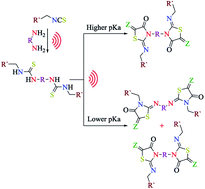
A novel series of asymmetrically substituted bis-thioureas has been synthesized in an effective pattern via the reaction of diamines with isothiocyanates utilizing the ultrasonic irradiation as a sustainable energy source. This reaction performs well at mild conditions to give the products in quantitative yields for a broad scope of substrates. The bis-thiourea derivatives are used for the design of unprecedented series of bis-2-iminothiazolidin-4-ones promoted by the ultrasonic irradiation. The reaction affords the formation of regioselective products, which depends on the pKas of the diamines. The diamine linked to the thiourea possessing lower pKa is involved in the imino part, and the diamine having higher pKa is a part of the other heterocyclic nitrogen. Moreover, this new strategy has excellent environmental parameters, demonstrating that this protocol is a green and sustainable process.


 Please wait while we load your content...
Please wait while we load your content...