Divergent synthesis of dual 1,4-dihydropyridines with different substituted patterns from enaminones and aldehydes through domino reactions†
Abstract
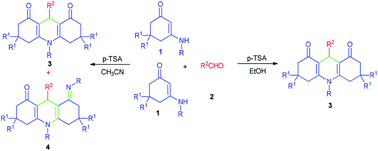
A concise and efficient protocol for the regioselective synthesis of dual 1,4-dihydropyridines with several substituted patterns has been developed from a cascade cyclization of enaminones and aldehydes in different media (EtOH/CH3CN). The one-pot cascade reaction involves at least five reactive sites and generates multiple C–C and C–N bonds. The established protocol explores the chemistry of enaminones by employing their three reactive sites. The method has several advantages including mild conditions, operational simplicity, and high bond-forming efficiency. It may offer promise in a variety of biochemical applications.


 Please wait while we load your content...
Please wait while we load your content...