Rational design of a new cytarabine-based prodrug for highly efficient oral delivery of cytarabine†
Abstract
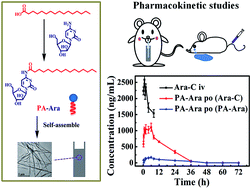
Because of the drawbacks of cytarabine (Ara-C) such as poor lipid solubility, deamination inactivation and low oral bioavailability limiting its application by oral administration, herein we propose a novel amphiphilic low molecular weight cytarabine prodrug (PA-Ara) by conjugating palmitic acid (PA) to Ara-C, making it possible to avoid the deamination inactivation by protecting the active 4-amino, as well as improving lipid solubility. Thanks to the rational design, the oil/water partition coefficient (P) of PA-Ara was improved tremendously compared with Ara-C, and the PA-Ara conjugation was stable enough in artificial digestive juice, ensuring that most molecules could be absorbed in the form of the prodrug. Results from an MTT assay conducted to measure the cytotoxicity of Ara-C and PA-Ara to HL60 (acute myeloblastic leukemia cell line) and K562 cells (chronic granulocytic leukemia cell line) showed that PA-Ara had significantly stronger antiproliferation activities than Ara-C. Significantly, we firstly compared the bioavailability of the oral fatty acid chain modified cytarabine prodrug preparation with injection and the relative bioavailability was up to 61.77% for our PA-Ara, which was much superior to that of oral Ara-C solution (3.23%). Overall, these findings make it clear that the PA-Ara suspension has the potential to be a promising new cytarabine oral preparation for leukemia therapy.


 Please wait while we load your content...
Please wait while we load your content...