Effects of multiwalled carbon nanotubes on CH4 hydrate in the presence of tetra-n-butyl ammonium bromide
Abstract
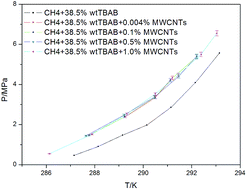
Hydrate formation is an important technology for gas storage and transportation. In this work, the effect of multiwalled carbon nanotubes (MWCNTs) on CH4 hydrate formation was examined by determining the phase equilibrium conditions and kinetics characteristics of a mixed system of CH4, tetra-n-butyl ammonium bromide (TBAB), and MWCNTs. The phase equilibrium was examined in the temperature range of 286.13–293.04 K and the pressure range of 0.55–6.56 MPa for various mass fractions of MWCNTs (0.004, 0.1, 0.5, and 1.0 wt%). In the CH4 + TBAB system, the presence of MWCNTs was found to shift the phase equilibrium conditions to a lower temperature by about 1 K compared with those in the absence of MWCNTs. However, the concentration of MWCNTs had little effect on the phase equilibrium conditions. When the concentration of MWCNTs was 1.0 wt%, the addition of MWCNTs reduced the induction time of hydrate formation by 79.5%. When the concentration of MWCNTs was 0.1 wt%, the addition of MWCNTs enhanced the hydrate growth rate by 61.5%. Powder X-ray diffraction patterns revealed that hydrates with orthorhombic structures (corresponding to TBAB·38H2O with 3D cages) were formed in the systems with and without MWCNTs. Moreover, peaks corresponding to MWCNTs were not observed in the patterns of the hydrates and the addition of MWCNTs had no influence on the structure and type of hydrate. Thus, MWCNTs were not incorporated into the hydrate cages.


 Please wait while we load your content...
Please wait while we load your content...