Efficient synthesis of ether phosphonates using trichloroacetimidate and acetate coupling methods
Abstract
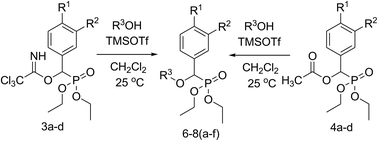
A series of ether phosphonates have been prepared by trichloroacetimidate and acetate coupling methods. Trichloroacetimidates or acetates were treated with primary and secondary alcohols as O-nucleophiles in the presence of catalytic TMSOTf to afford 21 examples of diethyl alkyloxy(substitutedphenyl)methyl phosphonates via C–O bond formation in 55–90% yields and short reaction time.


 Please wait while we load your content...
Please wait while we load your content...