Evaluating Ylehd, a recombinant epoxide hydrolase from Yarrowia lipolytica as a potential biocatalyst for the resolution of benzyl glycidyl ether†
Abstract
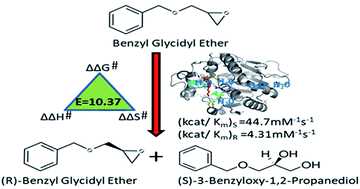
Glycidyl ethers and their vicinal diols are important building blocks in the organic synthesis of anti-cancer and anti-obesity drugs. Ylehd, an epoxide hydrolase from tropical marine yeast Yarrowia lipolytica, was explored for its enantioselective properties by kinetic, thermodynamic and in silico studies. Kinetic resolution of racemic phenyl glycidyl ether (PGE) yielded (S)-epoxide while for benzyl glycidyl ether (BGE) (R)-epoxide was obtained, with vicinal diols of the opposite configuration. Amongst the enantiomers of PGE and BGE, the (S)-selective conversion of benzyl glycidyl ether to its corresponding diol, (S)-3-benzyloxy-1,2-propanediol while retaining (R)-BGE was most favourable with 95% ee in 20 min. Enantioselective conversion of specific enantiomer of BGE to its corresponding diols was attributed to the favourable kinetic and thermodynamic parameters as well as to the number and proximity of water molecules near the base H325 in the active site pocket. The easily available and highly active Ylehd could be a potential biocatalyst for large scale preparation of pharmaceutically relevant chiral (R)-benzyl glycidyl ether and (S)-3-benzyloxy-1,2-propanediol.


 Please wait while we load your content...
Please wait while we load your content...