The effect of octahedral distortions on the electronic properties and magnetic interactions in O3 NaTMO2 compounds (TM = Ti–Ni & Zr–Pd)
Abstract
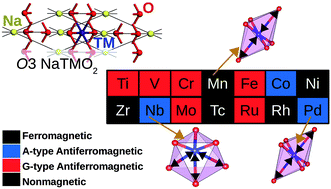
The interplay between the coordination environment and magnetic properties in O3 layered sodium transition metal oxides (NaTMO2) is a fascinating and complex problem. Through detailed and comprehensive density functional investigations on O3 NaTMO2 compounds, we demonstrate that the TM ions in O3 NaMnO2, NaFeO2 and NaCoO2 adopt a high spin state. Structurally, NaMnO2 and NaPdO2 undergo Jahn–Teller distortions while NaNbO2 undergoes puckering distortion. Furthermore, in addition to Jahn–Teller distortion, NaPdO2 exhibits charge disproportionation as it contains Pd2+, Pd3+ and Pd4+ species. These distortions stabilize the inter-plane ferromagnetism. Additionally, the inter-plane ferromagnetic coupling is stabilized by kinetic p–d exchange mechanism in undistorted NaCoO2, NaNiO2 and NaTcO2. The intra-plane coupling in this family of compounds on the other hand was found to be generally weak. Only NaMnO2, NaNiO2 and NaTcO2 are predicted to show bulk ferromagnetism with estimated Curie temperatures below ∼50 K.


 Please wait while we load your content...
Please wait while we load your content...