A novel strategy for the manufacture of idelalisib: controlling the formation of an enantiomer†
Abstract
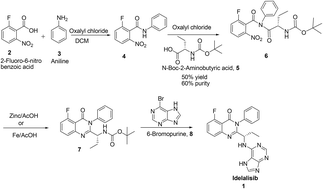
A novel and scalable synthesis of 5-fluoro-3-phenyl-2-[(1S)-1-(9H-purin-6-ylamino)propyl]-4(3H)-quinazolinone, idelalisib 1, has been developed. This strategy controls the desfluoro impurity of 13 during reduction of nitro intermediate 4, and also arrests the formation of the enantiomer during cyclisation of diamide 17, without affecting the neighbouring chiral centre. This process is demonstrated on a larger scale in the laboratory and achieved good chemical and chiral purities coupled with good yields.


 Please wait while we load your content...
Please wait while we load your content...