Rational design of fluorescent probe for Hg2+ by changing the chemical bond type†
Abstract
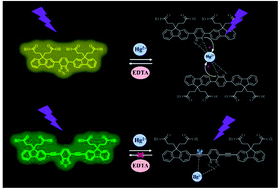
Two kinds of fluorescent probes DFBT and DFABT, and their corresponding water-soluble compounds WDFBT and WDFABT, based on the trimers containing a benzo[2,1,3]thiadiazole moiety and two fluorene moieties are synthesized. Their luminescent behavior towards Hg2+ ions and other various metal ions in organic and water solutions are studied in detail via absorption and emission spectroscopy. All these probes show a selective “on–off-type” fluorescent response to Hg2+ ions in solution over other metal ions with a maximum detection limit of 10−7 M. Importantly, the probe type can be changed from irreversible to reversible by altering the bridge mode between the functional units from C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bond to C–C single bond. Their detection mechanisms towards Hg2+ are studied in detail via mass spectrometry and Job plots, which are attributed to irreversible chemical reaction for DFABT and WDFABT and a reversible coordination reaction for DFBT and WDFBT respectively. Our research results about this kind of organic fluorescent probe provide valuable information to the future design of practical Hg2+ fluorescent probes.
C triple bond to C–C single bond. Their detection mechanisms towards Hg2+ are studied in detail via mass spectrometry and Job plots, which are attributed to irreversible chemical reaction for DFABT and WDFABT and a reversible coordination reaction for DFBT and WDFBT respectively. Our research results about this kind of organic fluorescent probe provide valuable information to the future design of practical Hg2+ fluorescent probes.


 Please wait while we load your content...
Please wait while we load your content...