Substituted l-tryptophan-l-phenyllactic acid conjugates produced by an endophytic fungus Aspergillus aculeatus using an OSMAC approach†
Abstract
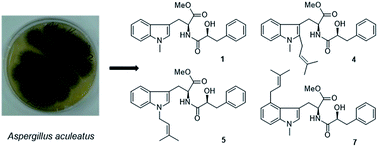
The endophytic fungus Aspergillus aculeatus isolated from leaves of the papaya plant Carica papaya was fermented on solid rice medium, yielding a new L-tryptophan-L-phenyllactic acid conjugate (1) and thirteen known compounds (11, 14–25). In addition, an OSMAC approach was employed by adding eight different sodium or ammonium salts to the rice medium. Addition of 3.5% NaNO3 caused a significant change of the metabolite pattern of the fungus as indicated by HPLC analysis. Subsequent isolation yielded several new substituted L-tryptophan-L-phenyllactic acid conjugates (1–10) in addition to three known compounds (11–13), among which compounds 2–10, 12–13 were not detected in the rice control culture. All structures were unambiguously elucidated by one and two dimensional NMR spectroscopy and by mass spectrometry. The absolute configuration of the new compounds was determined by Marfey's reaction and X-ray single crystal diffraction. Compounds 19–22 showed cytotoxicity against the L5178Y mouse lymphoma cell line with IC50 values of 3.4, 1.4, 7.3 and 23.7 μM, respectively.


 Please wait while we load your content...
Please wait while we load your content...