Lithium fluoride recovery from cathode material of spent lithium-ion battery
Abstract
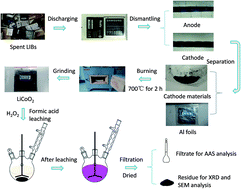
Recoveries of cobalt and lithium metals from spent lithium-ion batteries are very important for prevention of environmental pollution and alleviation of resource shortage. In this study, a hydrometallurgical route for the recovery of lithium fluoride was proposed. Lithium and cobalt could be first selectively leached into solution using formic acid and hydrogen peroxide. By investigating the effects of leaching temperature, time, stoichiometric ratio, H2O2 concentration and solid-to-liquid ratio, the leaching efficiency of Li and Co could reach 99.90% and 99.96%, respectively. Meanwhile, the evaluation of leaching kinetics and calculation of apparent activation energies revealed that the leaching process fitted chemical control satisfactorily. After further fractional precipitation, a high purity of 99.0% lithium fluoride could be finally obtained, thus achieving the effective recovery of spent material from the lithium-ion battery.


 Please wait while we load your content...
Please wait while we load your content...