Magnetic interactions and in vitro study of biocompatible hydrocaffeic acid-stabilized Fe–Pt clusters as MRI contrast agents†
Abstract
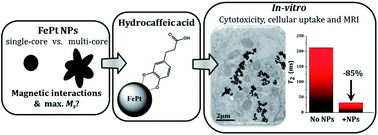
A detailed magnetic study of separated Fe–Pt NPs and Fe–Pt clusters was performed to predict their optimal size and morphology for the maximum saturation magnetization, a factor that is known to influence the performance of a magnetic-resonance-imaging (MRI) contrast agent. Excellent stability and biocompatibility of the nanoparticle suspension was achieved using a novel coating based on hydrocaffeic acid (HCA), which was confirmed with a detailed Fourier-transform infrared spectroscopy (FTIR) study. An in vitro study on a human-bladder papillary urothelial neoplasm RT4 cell line confirmed that HCA-Fe–Pt nanoparticles showed no cytotoxicity, even at a very high concentration (550 μg Fe–Pt per mL), with no delayed cytotoxic effect being detected. This indicates that the HCA coating provides excellent biocompatibility of the nanoparticles, which is a prerequisite for the material to be used as a safe contrast agent for MRI. The cellular uptake and internalization mechanism were studied using ICP-MS and TEM analyses. Furthermore, it was shown that even a very low concentration of Fe–Pt nanoparticles (<10 μg mL−1) in the cells is enough to decrease the T2 relaxation times by 70%. In terms of the MRI imaging, this means a large improvement in the contrast, even at a low nanoparticle concentration and an easier visualization of the tissues containing nanoparticles, proving that HCA-coated Fe–Pt nanoparticles have the potential to be used as an efficient and safe MRI contrast agent.


 Please wait while we load your content...
Please wait while we load your content...