Synthesis and physical properties of brominated hexacene and hole-transfer properties of thin-film transistors†
Abstract
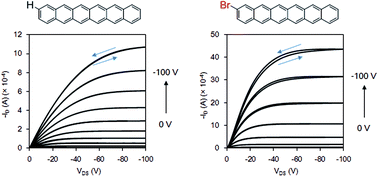
A halide-substituted higher acene, 2-bromohexacene, and its precursor with a carbonyl bridge moiety were synthesized. The precursor was synthesized through 7 steps in a total yield of 2.5%. The structure of precursor and thermally converted 2-bromohexacene were characterized by solid state NMR, IR, and absorption spectra, as well as by DFT computation analysis. It exhibited high stability in the solid state over 3 months, therefore can be utilized in the fabrication of opto-electronic devices. The organic thin-film transistors (OFETs) were fabricated by using 2-bromohexacene and parent hexacene through vaccum deposition method. The best film mobility of 2-bromohexacene was observed at 0.83 cm2 V−1 s−1 with an on/off ratio of 5.0 × 104 and a threshold of −52 V, while the best film mobility of hexacene was observed at 0.076 cm2 V−1 s−1 with an on/off ratio of 2.4 × 102 and a threshold of −21 V. AFM measurement of 2-bromohexacene showed smooth film formation. The averaged mobility of 2-bromohexacene is 8 fold higher than the non-substituted hexacene.


 Please wait while we load your content...
Please wait while we load your content...