Asymmetric synthesis of polysubstituted chiral chromans via an organocatalytic oxa-Michael-nitro-Michael domino reaction†‡
Abstract
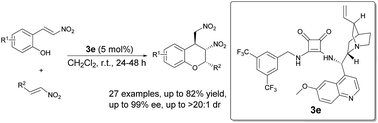
A catalytic asymmetric method for the synthesis of polysubstituted chromans via an oxa-Michael-nitro-Michael reaction has been developed. The squaramide-catalyzed domino reaction of 2-hydroxynitrostyrenes with trans-β-nitroolefins produced chiral chromans with excellent enantioselectivities (up to 99% ee), diastereoselectivities (up to >20 : 1 dr), and moderate to good yields (up to 82%).


 Please wait while we load your content...
Please wait while we load your content...