Insight into catalytic reduction of CO2 to methane with silanes using Brookhart's cationic Ir(iii) pincer complex†
Abstract
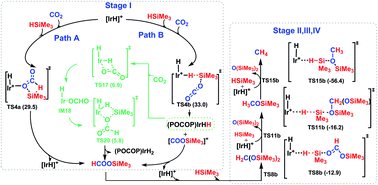
Using density functional theory computations, we investigated in detail the underlying reaction mechanism and crucial intermediates present during the reduction of carbon dioxide to methane with silanes, catalyzed by the cationic Ir-pincer complex ((POCOP)Ir(H)(acetone)+, POCOP = 2,6-bis(dibutylphosphinito)phenyl). Our study postulates a plausible catalytic cycle, which involves four stages, by sequentially transferring silane hydrogen to the CO2 molecule to give silylformate, bis(silyl)acetal, methoxysilane and the final product, methane. The first stage of reducing carbon dioxide to silylformate is the rate-determining step in the overall conversion, which occurs via the direct dissociation of the silane Si–H bond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of a weakly coordinated Ir–CO2 moiety, with a free energy barrier of 29.5 kcal mol−1. The ionic SN2 outer-sphere pathway in which the CO2 molecule nucleophilically attacks at the η1-silane iridium complex to cleave the η1-Si–H bond, followed by the hydride transferring from iridium dihydride [(POCOP)IrH2] to the cation [O
O bond of a weakly coordinated Ir–CO2 moiety, with a free energy barrier of 29.5 kcal mol−1. The ionic SN2 outer-sphere pathway in which the CO2 molecule nucleophilically attacks at the η1-silane iridium complex to cleave the η1-Si–H bond, followed by the hydride transferring from iridium dihydride [(POCOP)IrH2] to the cation [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OSiMe3]+, is a slightly less favorable pathway, with a free energy barrier of 33.0 kcal mol−1 in solvent. The subsequent three reducing steps follow similar pathways: the ionic SN2 outer-sphere process with silylformate, bis(silyl)acetal and methoxysilane substrates nucleophilically attacking the η1-silane iridium complex to give the ion pairs [(POCOP)IrH2] [HC(OSiMe3)2]+, [(POCOP)IrH2] [CH2(OSiMe3)2(SiMe3)]+, and [(POCOP)IrH2] [CH3O(SiMe3)2]+, respectively, followed by the hydride transfer process. The rate-limiting steps of the three reducing stages are calculated to possess free energy barriers of 12.2, 16.4 and 22.9 kcal mol−1, respectively. Furthermore, our study indicates that the natural iridium dihydride [(POCOP)IrH2] generated along the ionic SN2 outer-sphere pathway could greatly facilitate the silylation of CO2, with a potential energy barrier calculated at a low value of 16.7 kcal mol−1.
C–OSiMe3]+, is a slightly less favorable pathway, with a free energy barrier of 33.0 kcal mol−1 in solvent. The subsequent three reducing steps follow similar pathways: the ionic SN2 outer-sphere process with silylformate, bis(silyl)acetal and methoxysilane substrates nucleophilically attacking the η1-silane iridium complex to give the ion pairs [(POCOP)IrH2] [HC(OSiMe3)2]+, [(POCOP)IrH2] [CH2(OSiMe3)2(SiMe3)]+, and [(POCOP)IrH2] [CH3O(SiMe3)2]+, respectively, followed by the hydride transfer process. The rate-limiting steps of the three reducing stages are calculated to possess free energy barriers of 12.2, 16.4 and 22.9 kcal mol−1, respectively. Furthermore, our study indicates that the natural iridium dihydride [(POCOP)IrH2] generated along the ionic SN2 outer-sphere pathway could greatly facilitate the silylation of CO2, with a potential energy barrier calculated at a low value of 16.7 kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...