Synthesis of a series of benzothiazole amide derivatives and their biological evaluation as potent hemostatic agents†
Abstract
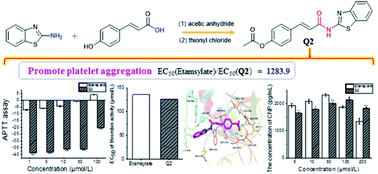
A series of benzothiazole amide derivatives were synthesized through a facile and efficient method via a nucleophilic acyl substitution reaction between 2-aminobenzothiazole and various cinnamic acid compounds. The obtained products exhibited good thermal stabilities. All compounds were evaluated for their in vitro hemostatic activities using the commercially available standard drug etamsylate as a positive control. The results showed that compound Q2 had a significant partial coagulation activity, reduced capillary permeability at 5, 10 and 50 μmol L−1, activated thrombin activity, and a more potent platelet aggregation activity than the positive control group (etamsylate, up to 1283.9 times in the nanomole range). A molecular modeling study revealed that compound Q2 was a competitive thrombin activator. Therefore, Q2 may be a potential lead for further biological screening and for the generation of drug molecules. Moreover, the structure–activity relationship of the prepared compounds is also discussed herein.


 Please wait while we load your content...
Please wait while we load your content...