Development of methodologies for the regioselective synthesis of four series of regioisomer isoxazoles from β-enamino diketones†
Abstract
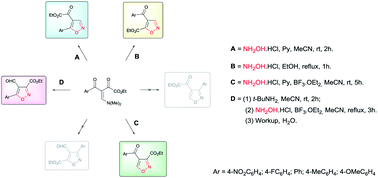
Four methodologies are reported for the regioselective synthesis of four series of regioisomer isoxazoles from cyclocondensation of β-enamino diketones and hydroxylamine hydrochloride. Regiochemical control was achieved by varying reaction conditions and substrate structure. The mild reaction conditions used to access 4,5-disubstituted, 3,4-disubtituted, and 3,4,5-trisubstituted regioisomer isoxazoles, as well as the pharmacological and synthetic potential of the products, make these novel methodologies very powerful.


 Please wait while we load your content...
Please wait while we load your content...