Insights into the mechanism of concomitant nucleation of form II and ethanol solvate of spironolactone in cooling crystallization†
Abstract
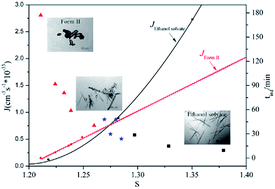
The concomitant crystallization of spironolactone form II and its ethanol solvate was investigated in ethanol by means of process analytical techniques, such as particle vision and measurement (PVM), focused beam reflectance measurement (FBRM) and Raman spectroscopy. The concomitant crystals were characterized by optical microscopy, powder X-ray diffraction, differential scanning calorimetry and thermogravimetric analysis. Analysis results of primary nucleation kinetics based on the experimental data of the induction time show that the ethanol solvate is the kinetically favored form with a lower interfacial energy, a higher nucleation rate and a smaller radius of the critical nucleus, compared with form II. At a high supersaturation, the crystallization process is dominated by kinetic factors and only the ethanol solvate is obtained. Whereas, at a low supersaturation, only form II crystallizes out owing to its thermodynamic priority. At a moderate supersaturation, concomitant crystals are found as a result of their nearly equal nucleation rates. In summary, the real cause for concomitant crystallization of form II and ethanol solvate of spironolactone is their simultaneous nucleation.


 Please wait while we load your content...
Please wait while we load your content...