Conservation of the conformational dynamics and ligand binding within M49 enzyme family†
Abstract
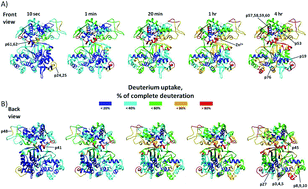
The hydrogen deuterium exchange (HDX) mass spectrometry combined with molecular dynamics (MD) simulations was employed to investigate conformational dynamics and ligand binding within the M49 family (dipeptidyl peptidase III family). Six dipeptidyl peptidase III (DPP III) orthologues, human, yeast, three bacterial and one plant (moss) were studied. According to the results, all orthologues seem to be quite compact wherein DPP III from the thermophile Caldithrix abyssi seems to be the most compact. The protected regions are located within the two domains core and the overall flexibility profile consistent with semi-closed conformation as the dominant protein form in solution. Besides conservation of conformational dynamics within the M49 family, we also investigated the ligand, pentapeptide tynorphin, binding. By comparing HDX data obtained for unliganded protein with those obtained for its complex with tynorphin it was found that the ligand binding mode is conserved within the family. Tynorphin binds within inter-domain cleft, close to the lower domain β-core and induces its stabilization in all orthologues. Docking combined with MD simulations revealed details of the protein flexibility as well as of the enzyme–ligand interactions.


 Please wait while we load your content...
Please wait while we load your content...