The behavior of the aluminum trimer when combining with different superatom clusters†
Abstract
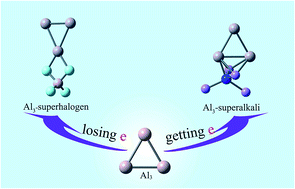
The interaction between the aluminum trimer and representative (super)halogens X (X = F, LiF2, BeF3, BF4) and (super)alkalis M (M = Li, FLi2, OLi3, NLi4) has been theoretically investigated at the MP2/6-311+(3df) level. Various geometrical structures were obtained for the resulting Al3–X and Al3–M superatom compounds, respectively. Natural bond orbital analysis reveals that the Al3 moiety exists in a cationic state in Al3–X while in an anionic state in Al3–M compounds. And the charge transfer between Al3 and (super)atoms is found to be enhanced in either polar or nonpolar solvent. The studied superatom compounds feature large bond energies, binding energies, and HOMO–LUMO gaps, which not only reflect their stability but indicate strong interactions between Al3 and (super)atoms. Although the solvent effect is not significant for the stability of Al3–X, the Al3–superalkali compounds can be better stabilized in the presence of solvent molecules. In addition, these superatom compounds exhibit aromaticity both in the gas phase and in solution.


 Please wait while we load your content...
Please wait while we load your content...