Catalytic reactivity of an iridium complex with a proton responsive N-donor ligand in CO2 hydrogenation to formate†
Abstract
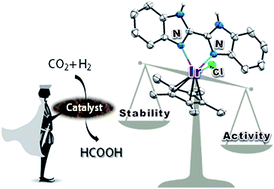
Catalytic hydrogenation of CO2 into formic acid/formate is an attractive conversion in the utilization of CO2. Although various catalysts with high catalytic efficiency are reported, a very few studies have been carried out to correlate/understand the efficacy and stability of the hydrogenation catalysts, which could be helpful to direct the future design strategy of corresponding catalysts. Herein, a half-sandwich iridium complex containing bibenzimidazole as a proton responsive N-donor ligand, [Cp*Ir(BiBzImH2)Cl]Cl, has been synthesized and fully characterized. The generation of an N− anion by the deprotonation of a bibenzimidazole group resulted in a significant enhancement of activity. The Ir complex showed about 20 times higher catalytic efficiency in the hydrogenation of CO2 into formate than that of its bipyridine counterpart [Cp*Ir(Bpy)Cl]Cl. The time dependent catalytic activity studies revealed that the initial excellent activity of [Cp*Ir(BiBzImH2)Cl]Cl was reduced when catalytic cycle proceeds; which was found to be the structural instability of the catalyst caused by steric hindrance between the bibenzimidazole and Cp* ligands.


 Please wait while we load your content...
Please wait while we load your content...