Biochemical characterization of a novel ulvan lyase from Pseudoalteromonas sp. strain PLSV†
Abstract
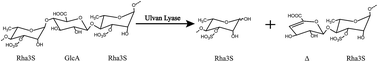
Ulvans, complex polysaccharides found in the ulvales (green seaweed) cell wall, contain predominantly 3-sulfated rhamnose (Rha3S) linked to either D-glucuronic acid, L-iduronic acid or D-xylose. The ulvan lyase endolytically cleaves the glycoside bond between Rha3S and uronic acid via a β-elimination mechanism. Ulvan lyase has been identified as belonging to the polysaccharide lyase family PL24 or PL25 in the carbohydrate active enzymes database, in which fewer members have been characterized. We present the cloning and characterization of a novel ulvan lyase from Pseudoalteromonas sp. strain PLSV (PsPL). The enzymes were heterologously expressed in Escherichia coli BL21 (DE3) and purified as the His-tag fusion protein using affinity chromatography, ion-exchange chromatography and size-exclusion chromatography. The degradation products were determined by thin-layer chromatography (TLC), liquid chromatography-mass spectrometry (LC-MS) to be mainly disaccharides and tetrasaccharides. Ulvan lyase provides an example of degrading ulvales into oligosaccharides. Arg265, His152 and Tyr249 were considered to serve as catalytic residues based on PsPL structural model analysis.


 Please wait while we load your content...
Please wait while we load your content...