Seawater splitting for hydrogen evolution by robust electrocatalysts from secondary M (M = Cr, Fe, Co, Ni, Mo) incorporated Pt
Abstract
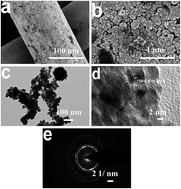
Water splitting is a promising technique for clean hydrogen energy harvesting. The creation of cost-effective electrocatalysts with improved hydrogen evolution reaction (HER) activity and stability is crucial in realizing persistent hydrogen evolution by reducing the reaction overpotential and minimizing energy consumption. Herein, we present the preparation of alloyed PtM (M = Cr, Fe, Co, Ni, Mo) modified titanium (Ti) mesh by a simple electrodeposition method, aiming at hydrogen generation from seawater splitting. The preliminary results indicate that the Ti/PtM electrodes feature markedly reduced onset overpotentials and Tafel slopes as well as significantly increased exchange current densities compared with pristine Pt electrodes, arising from the incorporation of secondary M atoms into the Pt lattice for alloying effects. Moreover, the competitive dissolution reaction between guest M species to Pt with Cl2 in seawater is beneficial for enhancing the long-term stability of resultant PtM alloy electrodes. The optimized PtMo alloy electrode maintains 91.13% of the initial current density upon 172 h operation in real seawater, making it promising in practical applications.


 Please wait while we load your content...
Please wait while we load your content...