Changing molecular conjugation with a phenazine acceptor for improvement of small molecule-based organic electronic memory performance†
Abstract
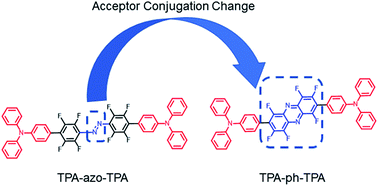
Two conjugated small molecules with different molecular conjugation, 4′,4′′-(diazene-1,2-diyl)bis(2′,3′,5′,6′-tetrafluoro-N,N-diphenyl-[1,1′-biphenyl]-4-amine) (TPA-azo-TPA) and 4,4′-(perfluorophenazine-2,7-diyl)bis(N,N-diphenylaniline) (TPA-ph-TPA), in which the electron donor triphenylamine moiety is bridged using different electron-accepting azobenzene or phenazine blocks, were designed and synthesized. The TPA-ph-TPA molecule with a larger conjugation acceptor regularly formed a nanocrystalline film and the as-fabricated memory devices exhibited outstanding non-volatile write once read many (WORM) memory effects with an ON/OFF ratio ten times higher than that of TPA-azo-TPA. Using theoretical calculations, it was speculated that the memory performance is a result of an electric field induced charge transfer effect and the enhanced device performance of the acceptor molecular conjugation is because of the presence of a strong charge transfer effect. The experimental findings suggest that the strategy of molecular conjugation may promote the performance of small molecule-based organic electronic memory devices by an enhanced a strong charge transfer effect.


 Please wait while we load your content...
Please wait while we load your content...