Gs protein peptidomimetics as allosteric modulators of the β2-adrenergic receptor†
Abstract
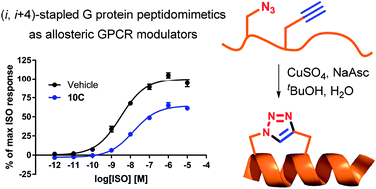
A series of Gs protein peptidomimetics were designed and synthesised based on the published X-ray crystal structure of the active state β2-adrenergic receptor (β2AR) in complex with the Gs protein (PDB 3SN6). We hypothesised that such peptidomimetics may function as allosteric modulators that target the intracellular Gs protein binding site of the β2AR. Peptidomimetics were designed to mimic the 15 residue C-terminal α-helix of the Gs protein and were pre-organised in a helical conformation by (i, i + 4)-stapling using copper catalysed azide alkyne cycloaddition. Linear and stapled peptidomimetics were analysed by circular dichroism (CD) and characterised in a membrane-based cAMP accumulation assay and in a bimane fluorescence assay on purified β2AR. Several peptidomimetics inhibited agonist isoproterenol (ISO) induced cAMP formation by lowering the ISO maximal efficacy up to 61%. Moreover, some peptidomimetics were found to significantly decrease the potency of ISO up to 39-fold. In the bimane fluorescence assay none of the tested peptidomimetics could stabilise an active-like conformation of β2AR. Overall, the obtained pharmacological data suggest that some of the peptidomimetics may be able to compete with the native Gs protein for the intracellular binding site to block ISO-induced cAMP formation, but are unable to stabilise an active-like receptor conformation.
- This article is part of the themed collection: Editors' collection: Chemical Biology


 Please wait while we load your content...
Please wait while we load your content...