Comparative pharmacokinetics of four active components on normal and diabetic rats after oral administration of Gandi capsules†
Abstract
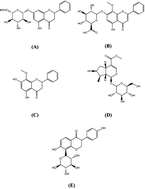
The Gandi capsule, a famous traditional Chinese medicine (TCM), is a hospital preparation that has been widely used in China for decades for the treatment of diabetes. The aim of this study is to compare the pharmacokinetics of four of the active components of Gandi capsules, which are the primary antidiabetic ingredients, on normal and diabetic Sprague-Dawley rats following oral administration of the capsules. Baicalin, wogonoside, wogonin, loganin and puerarin (internal standard) were prepared using methanol precipitation, and the separation of the five components was achieved through a ZORBAX Eclipse Plus C18 column by gradient elution using water (containing 0.1% formic acid) and acetonitrile as the mobile phase. After oral administration of Gandi capsules to the normal and diabetic rats, plasma was harvested and analyzed using liquid chromatography coupled with tandem mass spectrometry, and the primary pharmacokinetic parameters were calculated by DAS 2.0. Compared with the normal group, some pharmacokinetic parameters especially the AUC0–48 h of the four compounds significantly increased in the diabetic groups. The results demonstrated that the four constituents in normal and diabetic rats had obvious differences in some pharmacokinetic characteristics, suggesting that the rate and extent of drug metabolism were altered in diabetic animals. The results could be helpful for demonstrating the compatibility mechanism and providing clinical medication guidance for Gandi capsules.


 Please wait while we load your content...
Please wait while we load your content...