Polarization behavior of Pb–Co powder-pressed alloy for electrowinning
Abstract
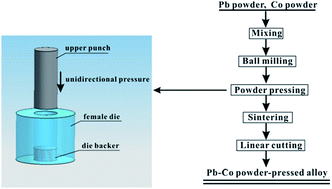
In this study, Pb–Co powder-pressed alloy was fabricated and used as a suitable anode material to replace Pb–Ca–Sn alloy in electrowinning. The Pb–Co anodes and the traditional Pb–Ca–Sn alloy on the electrochemical properties are investigated in a 160 g L−1 H2SO4 solution at 35 °C using galvanostatic polarization, electrochemical impedance spectroscopy and Tafel test. Thereafter, the anodic oxide layer is observed by energy dispersive X-ray spectroscopy along with scanning electron microscopy and X-ray diffractometry. The results show that the potential and oxygen evolution over-potential of the anodes exhibit a declining trend with increasing the fraction of Co. The anode potential of the Pb-2 wt% Co is approximately 170 mV lower than that of Pb–Ca–Sn alloy and reaches a stable value of 1.291 V at 35 °C, which shows good electrocatalytic performance and commercial application prospect.


 Please wait while we load your content...
Please wait while we load your content...