Two Cu(ii)-triadimenol complexes as potential fungicides: synergistic actions and DFT calculations†
Abstract
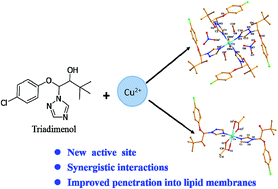
Two Cu(II) complexes, namely, [CuL4(H2O)2]·2NO3·2CH3OH 1 and [CuL2(CH3COO)2] 2, (L = (1S,2R)-1-(4-chlorophenoxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-ol, triadimenol, a commercial 1,2,4-triazole pesticide) were synthesized and characterized by elemental analysis, IR spectra, UV-Vis spectra and single crystal X-ray diffraction. Crystal structural analysis shows that the different types of salts (copper acetate is covalent, while copper nitrate is ionic) contribute to different crystal structures: complex 1 consists of one copper cation, four ligands, two coordinated water molecules, two free nitrate anions and two uncoordinated methanol molecules. Complex 2 is composed of one copper cation, two ligands and two acetate anions, without free molecules. The two complexes and the ligand triadimenol were also screened for antifungal activities against four selected fungi. The antifungal results reveal that both the complexes show better bioactivities in comparison with L, and that complex 1 has higher bioactivities than complex 2. To elaborate the reasons of the enhanced bioactivities after complexation, the interaction levels between Cu2+ cation and triadimenol, as well as the density functional theory (DFT) method were carried out. The results indicate that three factors made the antifungal activities stronger after forming Cu(II) complexes: new active site of copper cation, synergic interactions between Cu2+ cation and L, and improved penetration of the metal complexes into the lipid membranes.


 Please wait while we load your content...
Please wait while we load your content...