Improvement of O2 adsorption for α-MnO2 as an oxygen reduction catalyst by Zr4+ doping
Abstract
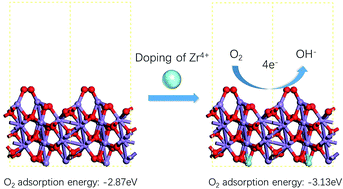
Zr4+ doped α-MnO2 nanowires were successfully synthesized by a hydrothermal method. XRD, SEM, TEM and XPS analyses indicated that Mn3+ ions, Mn4+ ions, Mn4+δ ions and Zr4+ ions co-existed in the crystal structure of synthesized Zr4+ doped α-MnO2 nanowires. Zr4+ ions occupied the positions originally belonging to elemental manganese in the crystal structure and resulted in a mutual action between Zr4+ ions and Mn3+ ions. The mutual action made Mn3+ ions tend to lose their electrons and Zr4+ ions tend to get electrons. Cathodic polarization analyses showed that the electrocatalytic activity of α-MnO2 for oxygen reduction reaction (ORR) was remarkably improved by Zr4+ doping and the Zr/Mn molar ratio notably affected the ORR performance of the air electrodes prepared by Zr4+ doped α-MnO2 nanowires. The highest ORR current density of the air electrodes prepared by Zr4+ doped α-MnO2 nanowires in alkaline solution appeared at Zr/Mn molar ratio of 1 : 110, which was 23% higher than those prepared by α-MnO2 nanowires. EIS analyses indicated that the adsorption process of O2 molecules on the surface of the air electrodes prepared by Zr4+ doped α-MnO2 nanowires was the rate-controlling step for ORR. The DFT calculations revealed that the mutual action between Zr4+ and Mn3+ in Zr4+ doped α-MnO2 nanowires enhanced the adsorption process of O2 molecules.


 Please wait while we load your content...
Please wait while we load your content...