Direct conjugate additions using aryl and alkyl organic halides in air and water†
Abstract
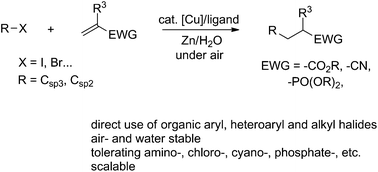
The direct aryl-conjugate addition to electron-deficient alkenes without prior stoichiometric formation of organometallic reagents in water catalyzed by copper represents an important but unresolved challenge. Herein, we describe a simple and convenient one-pot protocol for this type of conjugate addition. Compared with traditional methods that feature pre-formed stoichiometric organometallic reagents and strict anhydrous conditions, this transformation is actually performed in water and successfully applied to α,β-unsaturated esters, acrylonitriles and α,β-unsaturated phosphonates via direct arylation. Furthermore, this process is scalable and accommodates both aryl and alkyl halides. Importantly, the same reaction carried out in an organic solvent proceeded sluggishly to provide much inferior yields.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...