Kinetic resolution of 2,2-disubstituted-1,3-diketones via carbene catalysis†
Abstract
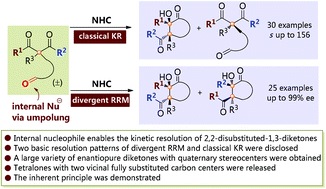
1,3-Diketones with quaternary stereocenters are extraordinarily useful building blocks both in academic research and industrial production. However, the access to enantiopure acyclic 2,2-disubstituted 1,3-diketones has suffered from an extremely limited substrate scope and poor stereoselectivity control. In this paper, we report the successful organocatalytic kinetic resolution of 1,3-diketones with central quaternary stereocenters through the introduction of internal nucleophiles. Two basic resolution modes were established, allowing access to a broad scope of enantioenriched 1,3-diketones with quaternary stereocenters, together with a large variety of tetralone derivatives with vicinal fully substituted carbon centers, and both of them are not easily available via currently known methods. The inherent principles between the different combinations of ketone groups and the resolution patterns were also disclosed. This work constitutes a good complementary choice for the construction of 1,3-diketones with quaternary stereogenic centers and provides insightful information for further studies on diketone substrate-mediated intramolecular annulations and kinetic resolutions.


 Please wait while we load your content...
Please wait while we load your content...