Mechanism and origins of chemo- and regioselectivities of (NHC)NiH-catalyzed cross-hydroalkenylation of vinyl ethers with α-olefins: a computational study†
Abstract
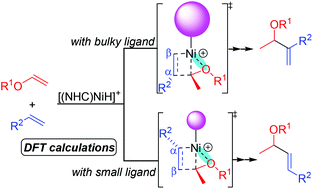
The (NHC)NiH-catalyzed cross-hydroalkenylation of vinyl ethers with α-olefins has been investigated by means of density functional theory calculations. The results show that the reaction is initiated by the hydrometallation of vinyl ether to afford a Ni-alkyl intermediate, from which the migratory insertion of α-olefin into the Ni–C bond leads to a five-membered alkyl nickel species. The catalytic cycle is closed by β-hydride elimination to generate the final cross-hydroalkenylation products. The computations reproduced quite well the experimentally observed chemo- and regioselectivities. The strong oxygen coordination to the Ni center resulting from the 2,1-hydrometallation of vinyl ether was found to be the key factor in controlling the chemo- and regioselectivities. The importance of the strong Ni–O interaction was found not only to promote the subsequent migratory insertion into the Ni–C bond, but also to stabilize the resulting five-membered alkyl nickel intermediate as the resting state of the overall reaction. The preference of α-olefin insertion into the Ni–C bond compared to vinyl ether is mainly because vinyl ether insertion into the Ni–C bond requires an additional energy barrier to break the p–π conjugation between the C–C double bond and the O atom. The bulkiness of the NHC ligands turns out to have an important impact on the regioselectivity. With a bulky ligand, the severe steric repulsion between the ligand and the α-substituent makes the α,β-insertion transition state less favored than the β,α-insertion transition state, leading to the formation of a tail-to-tail product. On the other hand, the steric repulsion around the forming C–C bond in the β,α-insertion transition state dominates the regioselectivity with a small ligand, which enables an experimentally observed selectivity switch upon the change of ligands.


 Please wait while we load your content...
Please wait while we load your content...