Maoeriocalysins A–D, four novel ent-kaurane diterpenoids from Isodon eriocalyx and their structure determination utilizing quantum chemical calculation in conjunction with quantitative interproton distance analysis†
Abstract
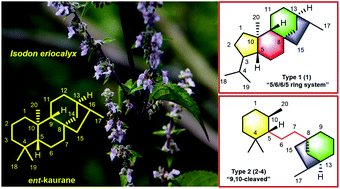
Maoeriocalysin A (1), a novel rearranged ent-kaurane diterpenoid with an unprecedented 4,5-seco-3,5-cyclo-7,20-epoxy-ent-kaurane scaffold, together with three rare 9,10-seco-7,20-epoxy-ent-kaurane diterpenoids, maoeriocalysins B–D (2–4), were isolated from the aerial parts of Isodon eriocalyx. Their structures were elucidated by comprehensive spectroscopic analysis, quantum chemical calculation of NMR parameters and electronic circular dichroism, quantitative interproton distance analysis, and single crystal X-ray diffraction experiment. The plausible biosynthetic pathway of 1–4 was also proposed.


 Please wait while we load your content...
Please wait while we load your content...