Mechanistic studies: enantioselective palladium(ii)-catalyzed intramolecular aminoarylation of alkenes by dual N–H and aryl C–H bond cleavage†
Abstract
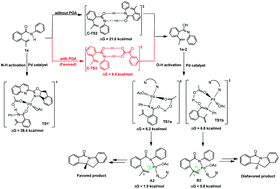
Nitrogen heterocyclic structures have been widely used in organic synthesis and medicinal chemistry. Recently Liu and co-workers reported an asymmetric palladium-catalyzed intramolecular oxidative aminoarylation of alkenes with quinoline–oxazoline (Qox) chiral ligands, and the products were formed in high yield with excellent enantioselectivity. The addition of a catalytic amount of phenylglyoxylic acid (PGA) significantly accelerates the reaction. However, it is hard to confirm the specific role of PGA experimentally. Herein, we provided DFT mechanistic insights to ascertain the mechanism of this reaction. The calculation results suggest that the barrier for the N–H deprotonation process of the amide substrate is extremely high, but the O–H deprotonation of its imidic acid tautomer is easier. The transformation of the amide to an imidic acid becomes more facile with the addition of PGA. The rate-determining step (with PGA) of this reaction is the reductive elimination and the stereoselectivity-determining step is alkene insertion. The enantioselectivity is essentially dominated by the steric repulsion between the chiral ligand Qox and the Pd–substrate complex.


 Please wait while we load your content...
Please wait while we load your content...