Acid-catalyzed oxidative cleavage of S–S and Se–Se bonds with DEAD: efficient access to sulfides and selenides†
Abstract
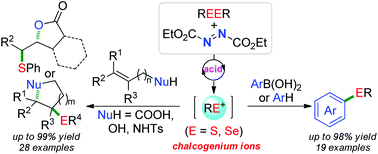
An acid/DEAD system for efficient oxidative cleavage of S–S and Se–Se bonds to generate chalcogenium ions has been developed. These ions could be captured by nucleophile-tethered alkenes and nucleophilic aryl reagents such as arylboronic acids and arenes to afford diverse chalcogenides. Mechanistic studies revealed that an N-sulfenylhydrazine intermediate was involved in the transformations.


 Please wait while we load your content...
Please wait while we load your content...