Transition-metal-free radical cleavage of a hydrazonyl N–S bond: tosyl radical-initiated cascade C(sp3)–OAr cleavage, sulfonyl rearrangement and atropisomeric cyclopropanation†
Abstract
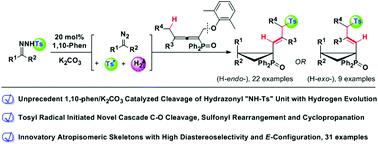
For the first time, the combination of catalytic 1,10-phenanthroline and potassium carbonate enables a radical cleavage of a hydrazonyl N–S bond to deliver a tosyl radical and a diazo compound, along with releasing molecular hydrogen spontaneously. The tosyl radical and hydrogen evolution are witnessed by radical trapping, EPR experiment and gas chromatography. An innovative application of this strategy into metal-free coupling of N-tosylhydrazone and phosphinyl allene provides a one-step synthesis of novel atropisomeric 3-tosyl-1-enyl-cyclopropyl-diphenylphosphine oxide derivatives with excellent diastereoselectivity and E-selectivity. Moreover, these atropisomeric products, verified by 1H-NMR, DFT calculations and X-ray crystallography, are controllably formed. Mechanistic studies indicate that the multistep cascade reaction occurs through radical hydrazonyl N–S bond cleavage, radical C(sp3)–OAr bond cleavage, sulfonyl rearrangement and atropisomeric cyclopropanation.
- This article is part of the themed collection: FOCUS: Radical-involved chemical transformations


 Please wait while we load your content...
Please wait while we load your content...