Lewis acid catalyst-steered divergent synthesis of functionalized vicinal amino alcohols and pyrroles from tertiary enamides†
Abstract
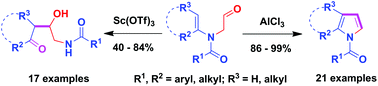
We report herein two Lewis acid catalyst-steered distinct reaction pathways of N-formylmethyl tertiary enamides. In the presence of a catalytic amount of AlCl3 and 4 Å molecular sieves, the stable N-formylmethyl tertiary enamides underwent sequential reactions involving intramolecular nucleophilic addition of tertiary enamides to aldehydes, isomerization and dehydration aromatization to produce diversely polysubstituted pyrroles in excellent yields. However, the Sc(OTf)3-catalyzed reaction of the same tertiary enamides proceeded through, after the initial enaminic addition to aldehydes, hydration of the cyclic iminium intermediates and the ring opening reaction to furnish functionalized vicinal amino alcohol derivatives in 40%–84% yields. The synthetic utility of the method was further demonstrated by the gram-scale preparation of di- and tri-substituted pyrroles and 1-acetyl-4,5,6,7-tetrahydro-1H-indole, and by a facile transformation of a functionalized pyrrole into 5H-pyrrolo[2,1-a]isoindol-5-one.


 Please wait while we load your content...
Please wait while we load your content...