Enantioselective and regiodivergent allylation of pyrimidines with terminal allenes: an approach to pyrimidine acyclic nucleosides†
Abstract
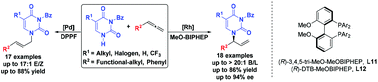
An atom-economic addition of pyrimidines to allenes has been developed for the diverse synthesis of branched or linear N-allylpyrimidine analogues. With [Rh(COD)Cl]2/chiral MeOBIPHEP as the catalyst, the asymmetric allylation reaction proceeded well and afforded the branch selective adducts in good yields, with high regio- and enantioselectivities. Meanwhile, when [Pd(η3-allyl)Cl]2/DPPF was used as the catalyst, the linear-selective allylation of pyrimidines could be carried out in good yields. Here, the configuration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the side chain of the products was mainly found to be the E-form.
C bond in the side chain of the products was mainly found to be the E-form.


 Please wait while we load your content...
Please wait while we load your content...