Selective modification of natural nucleophilic residues in peptides and proteins using arylpalladium complexes
Abstract
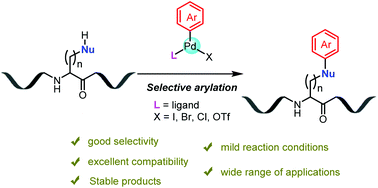
Transition metal-mediated modification of peptides and proteins is emerging as a powerful method for their selective functionalization and bioconjugation, particularly for native peptides and proteins bearing no unnatural bioorthogonal handles. The modified peptides and proteins are useful synthetic reagents needed in both biochemical and biophysical studies, as well as in pharmaceutical research. This mini-review surveys recent developments of regio- and chemoselective arylation reactions of the natural nucleophilic residues within unprotected peptides and proteins, promoted by arylpalladium complexes. These reactions exhibited high selectivities and excellent biocompatibility, proceeded under mild reaction conditions, and have a wide range of applications. They exemplify the advantages and potential of organometallic palladium complexes in bioconjugation, and are expected to inspire future studies on transition metal-mediated biocompatible modification reactions.
- This article is part of the themed collections: Celebrating the 90th birthday of Professor Lu Xiyan and 2018 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...