Striving to exploit alkyl electrophiles: challenge and choice in transition metal-catalyzed cross-coupling reactions of sulfones
Abstract
For cross-coupling reactions of alkyl electrophiles, alkyl halides, alkyl tosylates, alkyl acetates and alkyl sulfones exhibit high reaction efficiency when various transition metal catalysts and reaction partners are employed. As crystalline and readily available alkyl electrophiles, alkyl sulfones in radical cross-coupling reactions open a new avenue for modularly generating diverse alkyl compounds. It is believed that more transformations using sulfones as electrophiles will be developed in the near future.
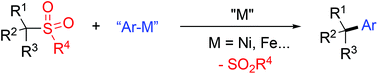
- This article is part of the themed collection: 2018 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...