One-pot diastereo- and enantioselective hydrosilylation–transacylation of α-acyloxy β-enamino esters†
Abstract
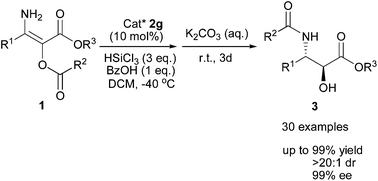
A one-pot diastereo- and enantioselective hydrosilylation–transacylation of N-unprotected α-acyloxy β-enamino esters was realized. By using an easily accessible novel chiral Lewis base as a catalyst, this transformation allowed for the direct synthesis of highly enantioenriched α-hydroxyl-β-acylamido esters (up to >20 : 1 dr and 99% ee). The absolute configurations of two products were assigned by comparison of the optical rotations with the literature and the absolute configurations of other products were determined accordingly.


 Please wait while we load your content...
Please wait while we load your content...