A computational mechanistic study of substrate-controlled competitive O–H and C–H insertion reactions catalyzed by dirhodium(ii) carbenoids: insight into the origin of chemoselectivity†
Abstract
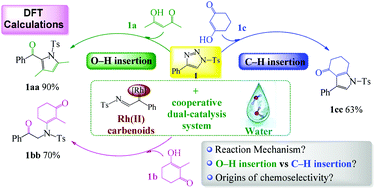
Density functional theory calculations were conducted to elucidate the mechanism, selectivity, and the origin of Rh2(oct)4 ([Rh])-catalyzed O–H/C–H insertion reactions of 1,3-diketones. For three different 1,3-diketone substrates (acetylacetone, 2-methylcyclohexane-1,3-dione, and cyclic 1,3-diketone), two different insertion modes O–H and C–H and three mechanisms were investigated under [Rh]-assisted, 2H2O-assisted, and [Rh]-nH2O (n = 1–3) co-assisted conditions. The computational results indicated that the [Rh]-2H2O co-assisted ones are the most favored cases. The corresponding rate-determining steps are the concerted C–N bond formation and H-shift process with an energy barrier of 20.4, 22.6, and 25.1 kcal mol−1, respectively. More importantly, it was found that water molecules can significantly improve the activity of the [Rh] catalyst and lower the free energy barrier. The distortion/interaction and natural bond orbital analyses suggested that the interaction energy is the dominant factor determining the difference of the reaction between three 1,3-diketones and the α-imino Rh(II) carbenoids. The chemoselectivity of the O–H/C–H insertion reactions of three different 1,3-diketone substrates depends on three factors: steric repulsion, the distortion of substrates, and electronic effects. Our findings can serve as a benchmark for other similar [Rh]-catalyzed reactions, which might open a new avenue for designing more efficient O–H/C–H insertion reactions.


 Please wait while we load your content...
Please wait while we load your content...