A Zn(OTf)2 catalyzed Ugi-type reaction of 3-(2-isocyanoethyl)indoles with indole-derived ketimines: rapid access to hexacyclic spiroindolines†
Abstract
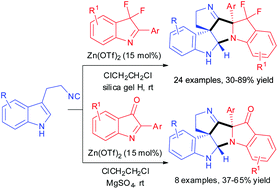
We report a Zn(OTf)2 catalyzed Ugi-type reaction of 3-(2-isocyanoethyl)indoles and indole-derived ketimines to rapidly afford hexacyclic spiroindolines featuring three stereocenters including two quaternary stereocenters in moderate to excellent yields (30–89%) with complete diastereoselectivity. This reaction is highly efficient because two C–C and one C–N bonds as well as two new rings are created under mild reaction conditions in a single step.


 Please wait while we load your content...
Please wait while we load your content...