Catalytic asymmetric 1,6-conjugate addition of in situ generated para-quinone methides with tritylthiol†
Abstract
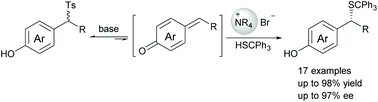
An asymmetric phase-transfer catalyzed 1,6-conjugate addition of in situ generated para-quinone methides (p-QMs) with tritylthiol has been developed, affording a range of optically active α-substituted benzyl thioethers in excellent yields (up to 98%) and enantioselectivities (up to 97% ee). The use of 4-hydroxybenzyl p-tolyl sulfones as precursors for the formation of p-QMs allows one to overcome the limitations in the substrate scope.


 Please wait while we load your content...
Please wait while we load your content...