Highly efficient, catalyst-free, one-pot, pseudo-seven-component synthesis of novel poly-substituted pyrazolyl-1,2-diazepine derivatives†
Abstract
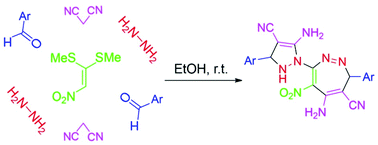
A novel, green and high yielding preparation of poly-substituted pyrazolyl-1,2-diazepine derivatives is described via a one-pot pseudo-seven-component condensation reaction under catalyst-free conditions in EtOH at room temperature. For this synthesis, at first, 1,1-bis(methylthio)-2-nitroethylene (BMTNE) was reacted with NH2NH2·H2O to form 1,1-dihydrazino-2-nitroethylene (DHNE). Then, the addition of aryl aldehydes and malononitrile afforded the corresponding products under aerobic oxidation in good to excellent yields. This synthetic procedure for the preparation of biologically important compounds provides several benefits such as high functional group tolerance, readily available starting materials, simplicity in operation and workup and mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...