Computational study on the NHC-catalyzed synthesis of 2,3-disubstituted indoles: mechanism, key intermediate and the role of the catalyst†
Abstract
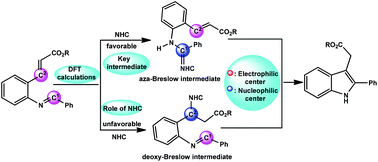
In this work, the possible reaction mechanisms and the role of N-heterocyclic carbene (NHC) catalysts in the umpolung reaction of imines to generate 2-substituted indole-3-acetic acid derivatives were investigated by density functional theory (DFT). Two different NHCs (named A-NHC and B-NHC) were studied as model catalysts. Calculations revealed that the whole reaction includes four steps: (1) the formation of a key aza-Breslow intermediate, (2) an intramolecular 1,4-addition, (3) a second 1,2-proton transfer, and (4) the desorption of the NHC to give 2-substituted indole-3-acetic acid derivatives. Furthermore, the nucleophilic attack of the NHC on the imine carbon or β-carbon of the α,β-unsaturated ester is the rate-limiting step that determines which intermediate is formed (the aza-Breslow or deoxy-Breslow intermediate). Analysis of the global reaction index indicates that NHCs play a vital role in the polarity reversal of an imine carbon atom or a β-carbon from the α,β-unsaturated ester. This strengthens the nucleophilicity of an imine carbon or a β-carbon from the α,β-unsaturated ester as a Lewis base. In addition, B-NHC has a slightly higher nucleophilicity value, which makes the reactivity of B-NHC slightly better than that of A-NHC. The theoretical results obtained not only rationalize the experimental observations well but also provide important insight into the mechanistic details of the reaction.


 Please wait while we load your content...
Please wait while we load your content...