Palladium-catalyzed CO-free cyclizative carbonylation of 2-benzylpyridines leading to pyridoisoquinolinones†
Abstract
A CO-free palladium-catalyzed cyclizative carbonylation of 2-benzylpyridines was developed, leading to pyridoisoquinolinones. This procedure proceeded with the sequential carbonylation of the ortho-C–H bond and the dearomative C–N bond formation. The combination of acetic anhydride and formic acid rather than toxic CO gas was employed in this reaction. The ortho-C–H palladation intermediate palladacycle, which showed comparable activity during this reaction, was well characterized. Notably, N-phenyl-2-pyridinamine also worked well to provide 11H-pyrido[2,1-b]quinazolin-11-one.
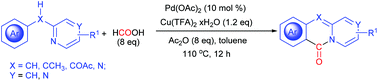


 Please wait while we load your content...
Please wait while we load your content...